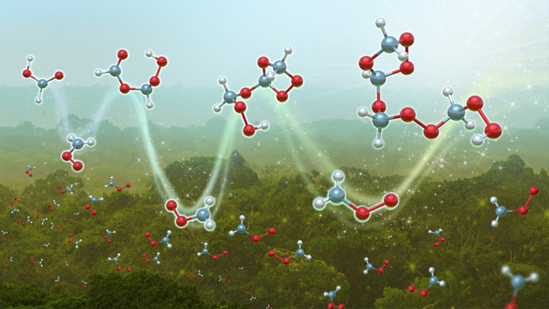
A multidisciplinary team has combined measurements in the Amazon forest with experimental and theoretical kinetics to show the role of Criegee intermediates (CIs) in forming secondary organic aerosols (SOAs). SOAs directly impact the planet’s radiation balance, air quality and human health. Reactions of CIs have been suspected to contribute to the formation of SOAs, but before these measurements, researchers had not identified the chemical signatures of this process in the field.
https://www.nature.com/articles/s41561-023-01361-6#citeas
Sandia researchers linked to work
Sponsored by
This material is based upon work supported by the Division of Chemical Sciences, Geosciences
and Biosciences, Office of Basic Energy Sciences (BES), U.S. Department of Energy (USDOE)
(RLC, ACR, AWJ, DLO, NH, SJK, CAT).
This research used resources of the Advanced Light Source, which is a DOE Office of Science
User Facility under contract no. DE-AC02-05CH11231. The contributions of RLC were in part supported by an appointment to the NASA Postdoctoral Program at the NASA Jet Propulsion
Laboratory, administered by Universities Space Research Association under contract with
NASA. This research was carried out in part by the Jet Propulsion Laboratory, California
Institute of Technology, under contract with the National Aeronautics and Space Administration
(NASA), supported by the Upper Atmosphere Research and Tropospheric Chemistry program
(FAFW, CJP). DES and MAHK were supported by NERC (NE/K004905/1), the Primary
Science Teaching Trust, and Bristol ChemLabS.
The fieldwork was supported by the FAPESP-University of Manchester SPRINT initiative (TJB, SDW, AB, MP, JDA, HC, CJP). PA acknowledges funding from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, 2017/17047-0 and 2023/04358-9). YJ was partly supported by the grants of DOE Plasma Science Center (DE-SC0020233) and National Science Foundation (CBET-1903362 and NSF-EFRI CBET-2029425).
IMT Nord Europe (JB) acknowledges financial support from the Labex CaPPA project, funded by the French National Research Agency (ANR, contract ANR-11-LABX-0005-01), and the CPER ECRIN, financed by the Regional Council “Hauts-de-France” and the European Regional Development Fund (ERDF).
This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not represent the views of the USDOE or the United States Government.
Figure 1 courtesy of Argonne National Laboratory, managed and operated by UChicago Argonne, LLC, for the U.S. Department of Energy under Contract No. DE-AC02-06CH11357.
The material has been authored by a contractor of the U.S. Government under Contract
No. DE-AC02-06CH11357. Accordingly, the U.S. Government retains for itself, and
others acting on its behalf, a paid-up, nonexclusive, irrevocable worldwide license in said article to reproduce, prepare derivative works, distribute copies to the public, and perform publicly and display publicly, by or on behalf of the Government.
Associated Publications
- Aric C. Rousso, Nils Hansen, Ahren W. Jasper, Yiguang Ju, “Identification of the Criegee intermediate reaction network in ethylene ozonolysis: impact on energy conversion strategies and atmospheric chemistry,” Phys. Chem. Chem. Phys. 21, 7341-7357 doi:10.1039/C9CP00473D (2019).
- Rabi Chhantyal-Pun, Brandon Rotavera, Max R. McGillen, M. Anwar H. Khan, Arkke J.Eskola, Rebecca L. Caravan, Lucy Blacker, David P. Tew, David L. Osborn, Carl J. Percival, Craig A. Taatjes, Dudley E. Shallcross, Andrew J. Orr-Ewing, “Criegee Intermediate Reactions with Carboxylic Acids: A Potential Source of Secondary Organic Aerosol in the Atmosphere,” ACS Earth Space Chem. 2, 833–842 doi:10.1021/acsearthspacechem.8b00069 (2018).
- Oliver Welz, Arkke J. Eskola, Leonid Sheps, Brandon Rotavera, John D. Savee, Adam M. Scheer, David L. Osborn, Douglas Lowe, A. Murray Booth, Ping Xiao, M. Anwar H. Khan, Carl J. Percival, Dudley E. Shallcross, and Craig A. Taatjes, “Rate Coefficients of C1 and C2 Criegee Intermediate Reactions with Formic and Acetic Acid Near the Collision Limit: Direct Kinetics Measurements and Atmospheric Implications,” Angew. Chem. Int. Ed. 53, 4547-4550 (2014).
- Oliver Welz, John D. Savee, David L. Osborn, Subith S. Vasu, Carl J. Percival, Dudley E.Shallcross, and Craig A. Taatjes, “Direct Kinetic Measurements of Criegee Intermediate(CH2OO) Formed by Reaction of CH2I with O2,” Science 335, 204-207doi:10.1126/science.1213229 (2012).
- Caravan, R.L., Bannan, T.J., Winiberg, F.A.F. et al. Observational evidence for Criegee intermediate oligomerization reactions relevant to aerosol formation in the troposphere. Nat. Geosci. 17, 219–226 (2024). https://doi.org/10.1038/s41561-023-01361-6
March 14, 2024