
Steel pipes rust and eventually fail. Therefore, oil companies and others have created computer models to predict when replacement is needed.
But if the models themselves go wrong, they can be modified only through experience, a costly problem if detection comes too late.
Now researchers at Sandia, the DOE Center for Integrated Nanotechnologies and the Aramco Research Center in Boston have found that a particular form of nanoscale corrosion can cause variations in material longevity that unpredictably decrease the working life of steel pipes, according to a recent paper in Nature’s Materials Degradation journal.
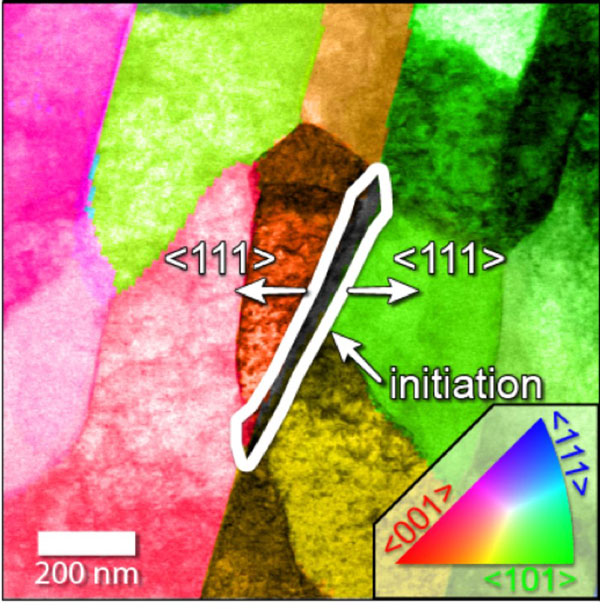
Using a variety of CINT’s transmission electron microscopes, which shoot electrons through targets to take pictures, the researchers were able to pin the root of the problem on a triple junction formed by a grain of cementite — a compound of carbon and iron — and two grains of ferrite, a type of iron. The junction frequently forms during most methods of fashioning steel pipe.
The researchers found that interfacial disorder in the atomic structure of those triple junctions made it easier for the corrosive solution to remove iron atoms along that interface.
In their experiments, the corrosive process stopped when the triple junction had been consumed by corrosion, but the crevice left behind allowed the corrosive solution to attack the interior of the steel.
“We thought of a possible solution for forming new pipe, based on changing the microstructure of the surface steel during forging, but it still needs to be tested and have a patent filed if it works,” said co-author and principal investigator Katherine Jungjohann, lead microscopist.
First observation of nanoscale corrosion
Aramco senior research scientist Steven Hayden added, “This was the world’s first real-time observation of nanoscale corrosion in a real-world material — carbon steel — which is the most prevalent type of steel used in infrastructure worldwide. Through it, we identified the types of interfaces and mechanisms that play a role in the initiation and progression of localized steel corrosion. The work is already being translated into models used to prevent corrosion-related catastrophes like infrastructure collapse and pipeline breaks.”
To mimic the chemical exposure of pipe in the field, where the expensive, delicate microscopes could not be moved, very thin pipe samples were exposed at Sandia to a variety of chemicals known to pass through oil pipelines.
Sandia researcher and co-author Khalid Hattar put dry samples in a vacuum and used a transmission electron microscope to create maps of the steel grain types and orientation, much as a pilot might use a camera to create aerial maps of farmland and roads — only Khalid’s maps had a resolution of about six nanometers, or six billionths of a meter.
“By comparing these maps before and after liquid corrosion experiments, a direct identification of the first phase that fell out of the sample could be identified, essentially identifying the weakest link in the internal microstructure,” Khalid said.
Co-author Paul Kotula said, “The sample we analyzed was considered a low-carbon steel, but it has relatively high-carbon inclusions of cementite, which are the sites of localized corrosion attacks.
Microscopes identified all the players
“Our transmission electron microscopes were a key piece of this work, allowing us to image the sample, observe the corrosion process and do microanalysis before and after the fact to identify the part played by the ferrite and cementite grains and the corrosion product,” Paul said.
Hayden said that when he started working in corrosion research, “I was daunted at how complex and poorly understood corrosion is. This is largely because realistic experiments would involve observing complex materials like steel in liquid environments and with nanoscale resolution, and the technology to accomplish such a feat had only recently been developed and had yet to be applied to corrosion.
“Now we are optimistic that further work at CINT and Sandia will allow us to rethink manufacturing processes to minimize the expression of the susceptible nanostructures that render the steel vulnerable to accelerated decay mechanisms.”
The invisible path of localized corrosion
Localized corrosion is different from uniform corrosion. The latter occurs in bulk form and is highly predictable. The former is invisible, creating a pathway observable only at its end point and increasing bulk corrosion rates by making it easier for corrosion to spread.
“A better understanding of the mechanisms by which corrosion initiates and progresses at these types of interfaces in steel will be key to mitigating corrosion-related losses,” the authors observed in their highly readable paper.
Other authors include Sandia researchers William Mook and Daniel Bufford, along with former Sandia researcher and experimental lead Claire Chisholm, now at the University of California at Santa Barbara.
The work was funded in part by Sandia’s Laboratory Directed Research and Development program. CINT is a DOE Office of Science user facility jointly operated by Sandia and Los Alamos national labs for university, industry and other national laboratory researchers.