Ionic liquid, or liquid salt, has a wide variety of uses, such as for solar power, batteries, carbon dioxide capture and pharmaceuticals. In addition to the sodium chloride we use in our favorite dishes, chemical salts include a vast array of other possible chemical combinations, such as cobalt nitrate and nickel chloride.
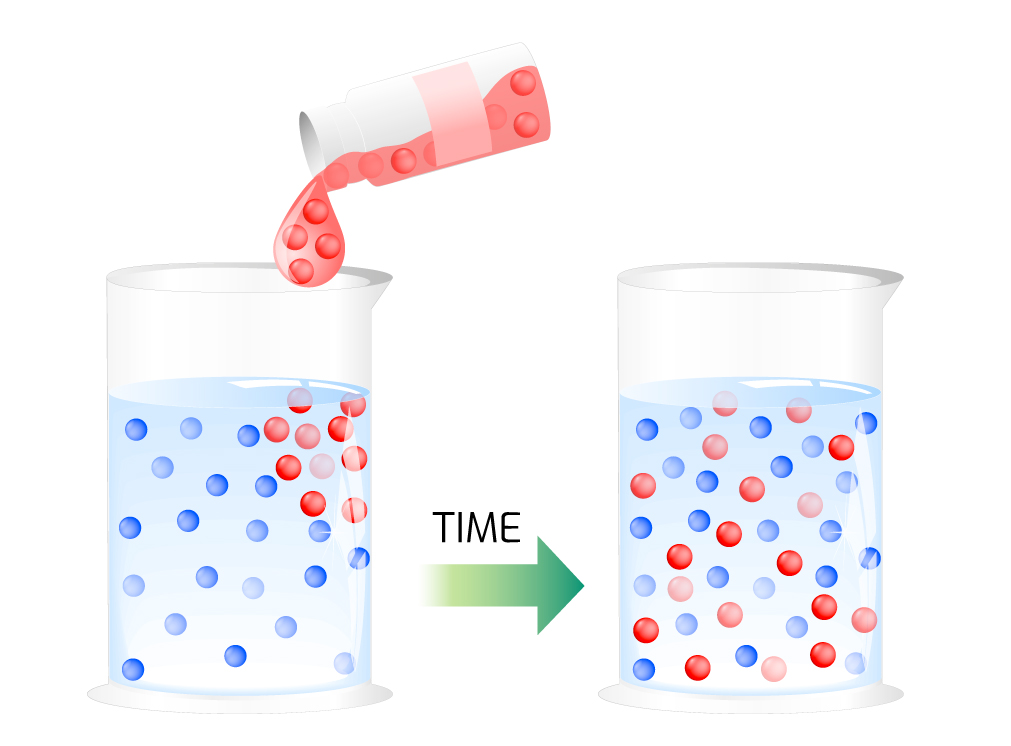
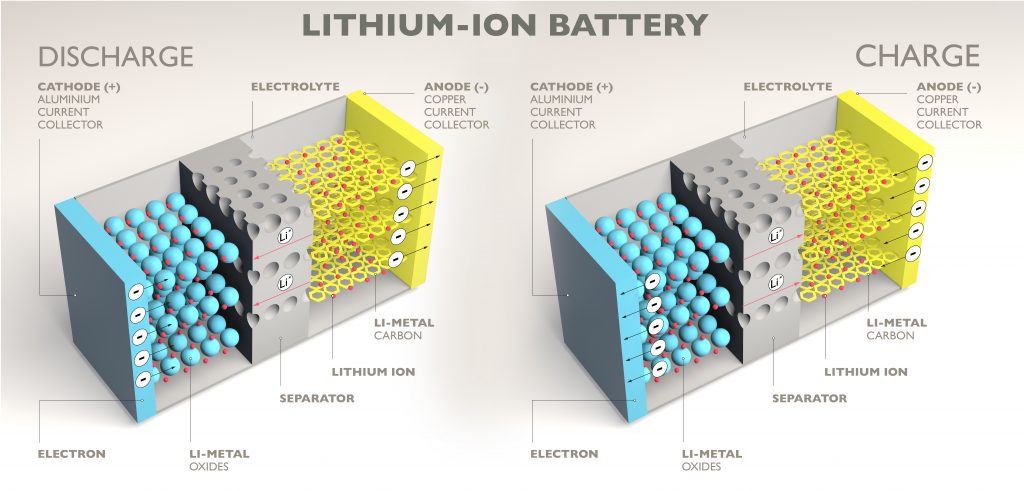
Diffusion is the intermingling of substances by the natural movement of their particles, typically shifting from a higher concentration in a small area to a lower concentration in a large area, for example, a drop of ink in a cup of water (see Figure 2). In liquid salts, diffusion describes the movement between positively charged molecules (cations) and negatively charged molecules (anions). This movement creates energy, for example, a lithium-ion battery powering a smartphone (see Figure 1).
Still, up to a trillion forms of ionic liquids have yet to be investigated to learn more about how they could be used to solve problems not only now but in the future. An enormous hurdle in these investigations is understanding how new types of ionic liquids will behave in various internal and external conditions. Since the behavior of ionic liquids is complex and non-linear, it’s extremely challenging to predict.
Sandia researchers began to tackle the problem by having the HPC computers use a combination of molecular dynamics simulations and machine learning methods to investigate 29 different ionic liquids in a controlled environment. They found that machine learning could accurately predict ion diffusion based on six to eight descriptive inputs, such as ion size, mass and geometry. This has proved to be a fast and inexpensive way to accurately predict diffusion behavior for ionic liquids, an important step forward in harnessing their full potential.