Sandia adds HIV and tuberculosis to rapid medical testing system SpinDx
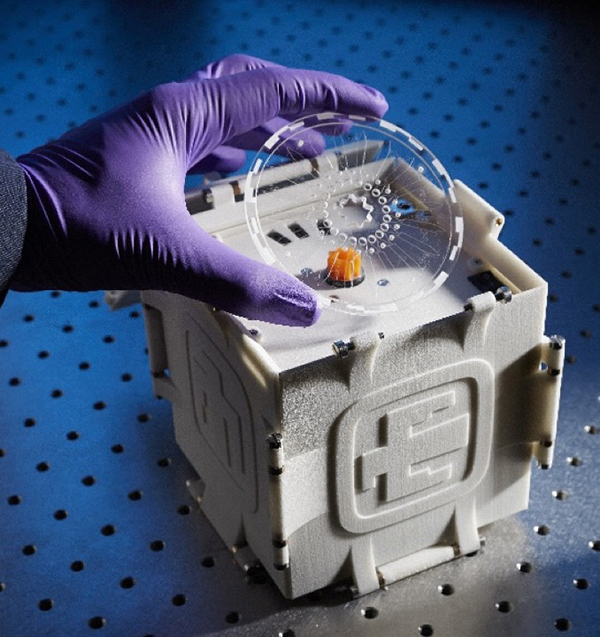
In less time than it takes most people to find their lost keys, Sandia’s SpinDx system can now simultaneously detect dozens of diseases including HIV and tuberculosis.
In a proof-of-principle study, Sandia scientists have demonstrated this SpinDx capability for the first time in a laboratory. Not only can the four-pound lab-on-a-disc system determine whether a patient has a current HIV or TB infection, it can determine whether a patient has ever been exposed to these illnesses.
Differentiating between latent and active infections is significant because it allows doctors to determine whether aggressive treatment measures are warranted.
Partnership enables progress
The SpinDx system evolved from earlier protein detection work conducted at Sandia by Sandia biochemist Chung-Yan Koh (8621) and his team. SpinDx already had the ability to detect an incredibly diverse portfolio of public health problems, including food-borne bacteria, toxins, markers of biological attacks, and levels of radiation in blood samples.
This work demonstrating HIV and TB detection using SpinDx was made possible through a grant from the National Institute of Allergy and Infectious Diseases and a collaboration with the University of Texas Medical Branch in Galveston.
Clinicians at the university were interested in new technology for rapid diagnosis of HIV and TB. They shared real patient samples that had already undergone several types of tests. Chung-Yan, working with the university’s Dr. Julia Litvinov and others, looked to see whether SpinDx could produce the same results as these prior tests, and it did.
“We are very fortunate that here in the United States tuberculosis is not very widespread. Because of this, out of the thousands of patients that move through the university hospital system we were only able to find three patient samples in two years that tested positive for both HIV and active infection with tuberculosis,” says Chung-Yan.
Smaller, more efficient
Although the ability to test for HIV and TB already exists, lab tests show SpinDx can do it less expensively, more quickly, and more reliably than the existing tests performed separately.
SpinDx looks and functions a bit like a DVD player. A disposable disc can send drops of raw, unprocessed biological samples into as many as 64 different channels that function, according to Chung-Yan, “like dozens of tiny test tubes.” The disc spins, and the samples interact with test chemistry or reagents inside the channels. If there is a reaction between the sample and the reagents, it will occur in about 15 minutes.
“You’re going to find a missing friend in a coffee shop a lot faster than you’re going to find them in a football stadium.”
Part of the reason SpinDx can give such rapid readouts is because its tests take place in extremely small spaces.
“By confining the space in which elements interact, things happen faster,” Chung-Yan explains. “You’re going to find a missing friend in a coffee shop a lot faster than you’re going to find them in a football stadium.”
Smaller space also means the system requires less sample. Typically, Chung-Yan says a doctor’s office will draw about three milliliters of blood. SpinDx only requires about 2 microliters. That is more than a thousand times less blood. One finger stick is sufficient to run any number of tests on the disc.
Microbeads allow major testing advantages
One common way to test for HIV and TB is through an enzyme-linked immunosorbent assay (ELISA) test. The ELISA technique can detect both active and previous exposure to illnesses, and is highly sensitive. The test uses antibodies, which are proteins manufactured by the immune system in response to infections.
In an ELISA test for an active illness, a slide is prepared with specific antibodies fixed to it. If the sample blood contains virus proteins, they will bind to the antibodies and give off a signal. In an ELISA test for signs of previous exposure to an illness, the slide would be prepared with antigens — proteins of a pathogen such as HIV or TB. The test would look for signs that antibodies in the blood have attached to the test antigen. At later stages of infections, when viruses have disappeared from the blood, the antibodies that flushed them are still detectable, making this a good way to diagnose previous exposures.
Though SpinDx uses an ELISA-type test, there is a key difference. ELISA test plates must be washed in between steps to prevent antibodies from sticking to elements in the blood that aren’t their targets. This labor-intensive requirement results in tests that take approximately three hours. SpinDx channels use microbeads in place of plates, and materials that filter out unwanted elements in the blood sample. No washing or sample preparation is required.
Similarly, polymerase chain reaction (PCR) tests are highly effective at disease detection. However, this method is also more labor intensive than SpinDx. PCR requires the extraction of DNA or RNA from a sample, then repeatedly heating and cooling the sample to cause any viral DNA/RNA to multiply to detectable levels.
“Typically, PCR tests are done in big clinical labs. They require highly skilled people with large machines. The robots that perform ELISAs in clinical labs are bigger, more complex, and even more expensive than PCR. We’ve been miniaturizing and simplifying testing processes for field use with SpinDx,” Chung-Yan says.
Better than eyeballs
Another key difference between SpinDx and other testing methods is the way results are indicated. Commonly used lateral flow tests are similar to home pregnancy tests: they require the application of a biological sample to a strip of paper pre-treated with antibodies. If the test antibodies bind to HIV or TB protein in the sample, the paper displays a color.
Smear tests, which are the gold standard for diagnosing TB in the field, similarly rely on the naked eye to detect pathogen presence. Smear tests require technicians to look for mycobacteria in phlegm samples under a microscope.
If there is a reaction in a SpinDx test, a red laser causes the tip of the microchannel to glow, signaling the presence of an infectious agent. The lasers interact with computer algorithms to determine a positive or negative result. When connected to a computer, SpinDx also supplies information about the amount of virus in a sample. The lasers and algorithms are inherently more sensitive than unaided vision alone.
Next steps
Now that Chung-Yan’s team has demonstrated these abilities in the laboratory, he says the next step is to test the system with more patient samples over a longer period of time. This will boost confidence in the reliability of SpinDx results for HIV and TB detection. “While our initial results are very encouraging, it is important to note that we tested only three patient samples and hence, more work needs to be done,” Chung-Yan says.
Commercial partners have licensed SpinDx technology in the past for widely different uses including male fertility tests, monitoring public water supplies for pathogens, and testing for drugs of addiction. Chung-Yan says he hopes that new partners will take the technology, mature the tests to move beyond proof-of-concept, and use it for disease detection in areas with limited resources.
“HIV and TB are still big public health concerns, and they’re hitting hardest in places that have the least ability to respond,” Chung-Yan says. “By making fast, mobile reliable detection methods more accessible we hope to have a positive impact on the problem.”
* * *
This work was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health (NIH) under Award Number R01AI098853. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.