Sandia discovers compounds in soot formation, findings could lead to cleaner engines
In most situations, breaking things apart isn’t the best way to solve a problem. However, sometimes the opposite is true if you’re trying to characterize complex chemical compounds. That’s what Sandia scientists Nils Hansen and Scott Skeen did to definitively identify soot precursor species in a flame.
The researchers discovered aliphatically bridged polycyclic aromatic hydrocarbons and PAHs with aliphatic side chains, which have been hypothesized to serve as “seeds” for soot particles in engine emissions. These are different variations of normal PAHs.
The newly recognized compounds can be used to create more detailed, up-to-date models of combustion that, in turn, can help in the design of cleaner, more efficient engines that emit less soot and fewer harmful hydrocarbons into the atmosphere.
“The role of these molecules as soot precursors has been hypothesized and there is indirect experimental evidence of their presence on the surface of soot extracted from flames,” Scott said. “Until now, however, no one had definitive experimental proof of their existence in the flame’s gas phase.”
Working with former Sandia postdoctoral researcher Brian Adamson and Musa Ahmed of Lawrence Berkeley National Laboratory, Nils and Scott recently published their discovery in the Journal of Physical Chemistry A. Funding for the research came from Sandia’s Laboratory Directed Research and Development program, while Ahmed is supported by the DOE’s Office of Basic Energy Sciences.
The team used an analytical technique called tandem mass spectrometry — using an instrument provided by Lawrence Berkeley Lab and cleverly customized by Adamson — to identify polycyclic aromatic hydrocarbons in flames that produce soot, something never done previously.
The device removes an electron to positively charge, or ionize, large molecules sampled from the flame, measures the molecule’s masses then further identifies the chemical structure by the way the ionized molecules crack apart.
Discovery builds on recent Sandia research
Recent work by Sandia scientist Hope Michelsen, technologist Paul Schrader and former postdoctoral researcher Olof Johansson broke ground by demonstrating chemical chain reaction processes in which hydrocarbons could form in soot. That work heightened the challenge of detecting and characterizing the compounds that participate in these processes.
One area of debate is whether the chemical byproducts in soot are purely polycyclic aromatic hydrocarbons, made up of ring-shaped groups of atoms, or contain extra, non-cyclic structures called alkyl, or aliphatic, chains. These long hydrocarbon chains can make the links among polycyclic aromatic hydrocarbons more stable at the high temperatures of combustion, greater than 2,000 degrees Celsius.
“Without the tandem component of this new mass spectrometer, each molecule’s mass is obtained but no information about its structure is revealed. You see something at mass 78, at mass 128, etc., but you don’t know what it is. You just use your chemical intuition,” Nils said. “Think of a mass spectrometer as an instrument that sorts a container full of mixed nuts based on the weight of each individual nut, but at the end, you still don’t know if you sorted peanuts, hazelnuts or walnuts.”
Breaking big molecules
The customized tandem mass spectrometer that the team used makes it easier to characterize the structure of large molecules by breaking them apart through high-energy collisions in a collision-induced dissociation cell, Scott said.
“Normal mass spectrometry can tell you how many atoms of each element are present in a molecule, but it won’t tell you anything about how those atoms are joined together,” Adamson said. “Tandem mass spectrometry with collision-induced dissociation isolates molecules of a single mass and then breaks them apart. The way they break apart provides clues as to the structure of the parent molecule.”
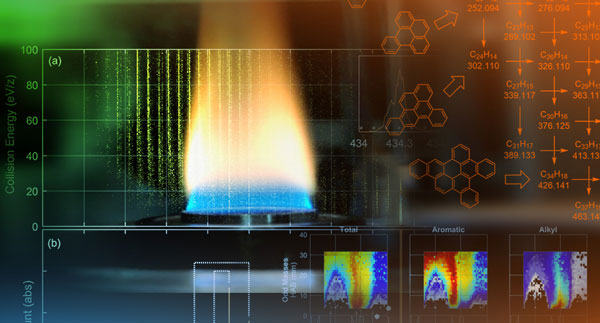
The team found direct evidence that polycyclic aromatic hydrocarbons and PAHs with alkyl chains exist in the sampled gases of the sooting flame. Such species may be sufficiently stable at the high temperatures of combustion to serve as key components in incipient soot particle formation.
The team also used a special flame configuration to minimize disruptions to the flame chemistry caused by the sampling process. Scott said the novel experimental setup involved sampling and examining large molecules from an inverted candle-like flame.
“In a candle, the wax moves up the wick and then vaporizes before burning in the surrounding air. The flame appears yellow because soot particles get very hot as they move through the flame,” Scott said. “In this configuration, it is impossible to sample soot particles or molecules that lead to soot formation without disturbing the flame because a probe must be inserted through the flame sheet.
“To overcome this problem, we generated a flame in which the air is in the center of the flame with the fuel on the outside,” he said. “This way, we can probe the gases of interest from the outside of this ‘inverse’ flame. This is perhaps the first time that such a flame has been attached to a tandem mass spectrometer.”
Engines creating solid soot from gas
In an engine, soot particles form when gaseous, carbon-containing molecules that originate from the fuel escape oxidation and combine to create larger molecules that eventually turn into a solid material.
Soot is harmful to the environment and a significant contributor to global climate change. Moreover, it impacts public health by damaging the lungs. Substantial evidence links polycyclic aromatic hydrocarbons and soot formation, although the evidence isn’t completely conclusive.
The chemical components leading to soot are difficult to decipher. What is known is that when the chemicals in hydrocarbon fuels are broken down during combustion, new molecules form rapidly.
The search for soot precursors is motivated by the need for cleaner engines that still run efficiently. Under certain driving conditions, diesel emissions exceed government regulations. This has led to the use of particulate filters that effectively capture soot particles from diesel exhaust, but they make the vehicles significantly more expensive and less efficient. Engines that produce less soot would need smaller particulate filters, reducing costs and increasing fuel economy.
Engine manufacturers typically use computer simulations to improve engine designs. They model the fuel injection, combustion and pollutant formation processes. Scott said that better understanding of how soot compounds are produced — specifically definitive identification of polycyclic aromatic hydrocarbons with alkyl chains attached — should lead to models that more accurately describe the effects of engine design parameters on emissions and efficiency.
“If we can understand the chemistry, we can develop a model that will allow engine designers to optimize fuel injectors, air flows and the shape of internal engine surfaces, among other things, that will keep these compounds out of the atmosphere,” Scott said.
Future steps
This discovery of alkyl-substituted and aliphatically bridged PAHs in sooting flames is only the starting point for using tandem mass spectrometry to decipher the complex chemistry of polluting emissions, the team said.
In the published paper, the team analyzed compounds at two different masses. However, the technique potentially could lead to identification of thousands of different types of compounds. Even for the most basic polycyclic aromatic hydrocarbons, there are about a hundred different ways the atoms can come together. Seeing all the different arrangements presents a formidable challenge. Musa will continue his work with Sandia scientists and plans to use complementary methods such as infrared spectroscopy for less ambiguous identification of alkyl-substituted and aliphatically-bridged PAHs in soot.
The Sandia scientists hope to collaborate with data scientists to develop more efficient, realistic models of engine soot formation, ultimately leading to designs for cleaner, more efficient engines.