Magnetic nanoparticles leap from lab bench to breast cancer clinical trials
Longstanding Sandia, industry collaboration produces precise particles
Dale Huber has been working on the challenge of making iron-based nanoparticles the exact same size for 15 years.
Now, the Sandia materials chemist and his long-term collaborators at Imagion Biosystems will use these magnetic nanoparticles for their first breast cancer clinical trial later this year. The nanoparticles — so small 3 billion of them would fit on the head of a pin with room to spare — stick to breast cancer cells, allowing the detection and removal of even small metastases.
Imagion Biosystems and Dale have been working together synthesizing nanoparticles since the opening of the Center for Integrated Nanotechnologies in 2006.
“Having access to the talent pool at CINT with experts like Dale Huber has been helpful,” said Bob Proulx, CEO of Imagion Biosystems. “Additionally, the fact that CINT has a user program that allows industry to access the facilities and equipment that, otherwise, would be too expensive for a small company like ours was valuable. The initial work we did with CINT to develop a method to give precise control over the size of the nanoparticle was key for our MagSense magnetic relaxometry technology for the detection of cancer.”
CINT is a user facility jointly operated by Sandia and Los Alamos National Laboratory for the U.S. DOE ’s Office of Science. CINT provides free access to state-of-the-art equipment and world-leading scientists for nanoscience researchers in academia and industry, provided they publish the results in scientific journals.
Producing precise particles
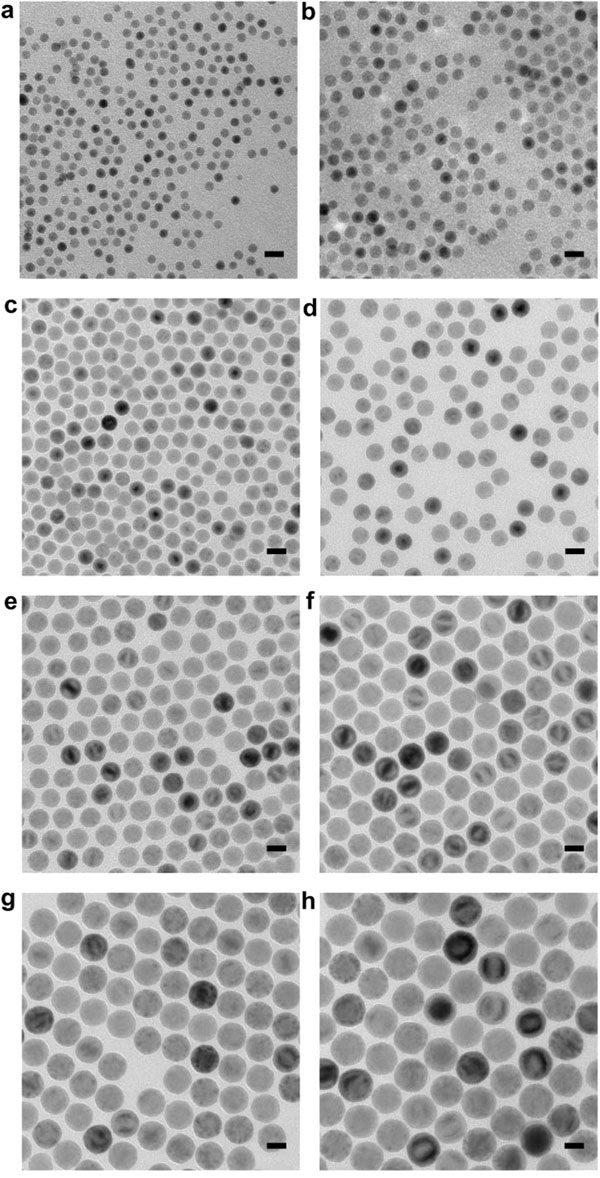
By changing the “cooking” time, Dale Huber and Imagion Biosystems can make nanoparticles to order. Transmission electron microscopy images of eight different batches of nanoparticles ranging in size from 9 nm (a) to 34.5 nm (h). (Image courtesy of Sandia National Laboratories)
The magnetic nanoparticles are coated with cancer antibodies, which stick specifically to cancerous cells. A tiny magnetic pulse — about the strength of a refrigerator magnet and hundreds of times weaker than one produced by an MRI machine — can sense the difference between nanoparticles stuck to cancer cells and those that are floating freely, allowing the detection of very small metastases.
Precision synthesis of magnetic nanoparticles
However, for Imagion Biosystems’ cancer detection method to work, all the nanoparticles have to be almost exactly the same size.
“A 2 percent variation is the difference between perfect and just about useless,” said Dale. He added laughing, “It was eye opening for me and if had I known that in the beginning, I might not have taken on the challenge.”
Erika Vreeland, who worked with Dale during her doctoral thesis to develop reproducible synthesis and was hired by Imagion Biosystems to be their chief nanoparticle scientist after she graduated said, “We eliminated all of the witchcraft of the reaction.”
The standard method to make iron nanoparticles is to combine the ingredients and heat the mixture to about 650 degrees Fahrenheit. How quickly the heat increases determines the nanoparticle size, said Dale. However, just like your oven at home, it will overshoot the critical temperature and then cool down until it levels off. How much the temperature overshoots this critical temperature also affects the size, producing nanoparticles more than 15 percent larger or smaller.
Instead, Dale and Vreeland developed a method where they slowly add the ingredients to a molten metal bath whose temperature varies less than half a degree. This produces nanoparticles with less than 2 percent size variation. Dale said, “It’s not the easiest way to make particles, but that’s why they’re so much better.”
Not only did the team discover a highly reproducible method to make the tiny particles, they also transferred the process twice — once to Imagion and once to ChemConnection, a nanoparticle manufacturer in the Netherlands that can make the nanoparticles under the strict U.S. Food and Drug Administration and European Union regulations needed for use in patient clinical trials.
“The synthesis was transferred to the lab in the Netherlands while maintaining size control,” said Dale. “This is huge. Everything changes, even the boiling points, because the Netherlands is basically at sea level.”
Clinical trial to detect spread of breast cancer this fall
After ChemConnection makes several batches, Imagion Biosystems will perform some preclinical trials to double-check the particles aren’t toxic. Then ChemConnection will make a small production lot of nanoparticles — comparable to a half teaspoon of sugar — for Imagion Biosystems’ breast cancer clinical trials.
“Because the nanoparticles are uniform and have excellent magnetic properties, we don’t need a lot. We expect that a patient will be injected with at most 1 milligram of particles,” said Vreeland.
All of the patients for the first clinical trial will be selected because their oncologists’ treatment regimen includes lymph node removal and biopsy. Before each patient has several lymph nodes removed surgically, the magnetic nanoparticles, coated in the breast cancer-specific antibodies, will be injected at the site of the known tumors. After the removal but before the biopsy, Imagion Biosystems’ detection system will examine removed lymph nodes to look for the spread of cancer.
Vreeland said she hopes Imagion Biosystems’ method will be as accurate as a pathologist, with the eventual goal of using this method first to look for cancer and eliminate the need to remove cancer-free lymph nodes.
“Our No. 1 aspiration is to see the nanoparticles make it into regular clinical use with our MagSense cancer detection technology. Beyond that we believe the nanoparticles can be instrumental in a wide variety of biomedical applications including uses in treatment of cancer or other diseases,” said Proulx.
Continuing collaboration to characterize nanoparticles and solve problems
CINT and Imagion Biosystems continued the collaboration beyond the effort to produce identically sized magnetic nanoparticles. Vreeland said, “We still run into all sorts of issues all the time so being able to talk with Dale or other scientists about some of the challenges we’re facing is really invaluable.”
Sandia bioengineer George Bachand assisted with the early toxicology and cell-targeting studies. Sandia researcher John Reno helped characterize the size and shape of the nanoparticles, using small angle X-ray scattering.
Small angle X-ray scattering is a method to determine the size and size distribution of nanoscale materials. “With CINT’s X-ray scattering instrument we can figure out exactly how big the particles are in 15 minutes. Seven or eight years ago it would take a week to figure out the same thing using electron microscopy,” said Dale.
This almost real-time size measurement enabled Vreeland to predict how the reaction would end and validate that they were on the right path, she said. The team used other CINT instruments to characterize the magnetic strength and coatings of the nanoparticles.
In addition to accessing the CINT experts and equipment through its user program, the partnership with Imagion Biosystems was supported by several New Mexico Small Business Assistance Program grants, which can support proprietary research.
The team has published several papers from the collaboration including one in Chemistry of Materials in 2015.