Ingenious method enables sharper images at lower energy costs
A perpetual quest of manufacturers and viewers is for ever-brighter colors and better images for flat-panel displays built from less expensive materials that also use less electricity.
An intriguing method discovered by Sandia researcher Alec Talin (8342) and collaborators at the Center for Nanoscale Science and Technology at the National Institute of Standards and Technology may be that next step. It uses super-thin layers of inexpensive electrochromic polymers to generate bright colors that, for the first time, can be altered rapidly. The work was reported in the Jan. 27 Nature Communications.
Electrochromic polymers by themselves are not a new invention. They change color in response to an applied voltage and only require energy when switched between colored and transparent states. But until Alec and his collaborators, no one had figured out how to switch electrochromics on and off in the milliseconds required to create moving images.
Long diffusion times a challenge
The problem lay in the thickness of the polymer. Conventional electrochromic displays require thick polymer layers in order to obtain good contrast between bright and dark pixels. But thick layers also require long diffusion times for ions and electrons to change the polymer’s charge state, making them only useful for static information displays or darkening windows of a Boeing Dreamliner, but not in the milliseconds needed for an action flick or even a roundtable discussion. On top of that, a full color display requires three different polymers.
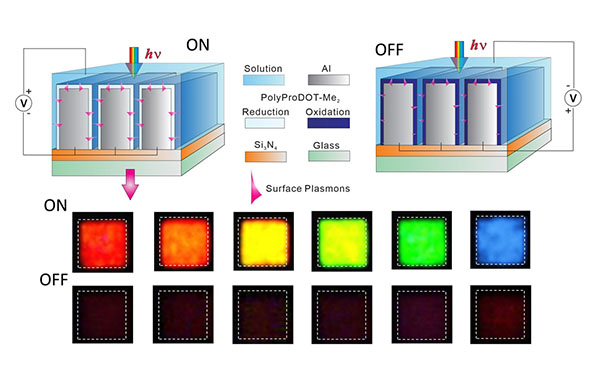
The electrochromic polymer turns transparent when charged by an electric pulse, allowing wavelengths of red, blue, or green (depending on the distance of the slit separation) to pass through the nanoslits cut in the aluminum track. When the pulse is turned off, the electrochromic material absorbs the surface plasmons and the pixel turns a rich dark black.
The researchers got around the rapidity problem with a tiny but spectacular innovation: They created arrays of vertical nanoscale slits perpendicular to the direction of the incoming light. The slits were cut into a very thin aluminum track coated with an electrochromic polymer. When light hit the aluminum nanoslits, it was converted into surface plasmon polaritons (SPPs), which are electromagnetic waves containing frequencies ranging from ultraviolet to infrared that travel along the dielectric interfaces — here, of aluminum and electrochromic polymer.
The distance between the slits in each array (pitch) corresponded exactly to the wavelengths of red, green, and blue light. The pitch determined which wavelength — red, blue, or green — was transmitted down through the array, traveling along the interface between the thin polymer layer and the aluminum substrate.
Because the polymer was just nanometers thick it required very little time to change its state of charge and, therefore, its optical absorption of colored light.
However, because the light traveled a relatively long distance down the surface of the aluminum slits coated with the thin polymer, it saw a much thicker polymer layer.
The material turned a desirable deep black when a tiny electric current sent across the top of the slit cut off the entering light, and did so in milliseconds. When the current was flicked off, light frequencies passed through the slits and instantly turned on the pixel. As an additional bonus, because the carefully spaced slits let in light only at a particular frequency, a single kind of polymer coating could serve as a neutral party and delivered all three emanated colors.
“These very inexpensive, bright, low-energy pixels can be turned on and off in milliseconds, making them fit candidates to provide improved viewing on future generations of screens and displays,” says Alec. “The nanoslits improve the optical contrast in a thin electrochromic layer from approximately 10 percent to over 80 percent.”
The work was supported at Sandia by Nanostructures for Electrical Energy Storage, a DOE Energy Frontiers Research Center.