Labs discoverers lend Sandia name — Sandia Octahedral Molecular Sieves — to a new class of ion catchers
Researchers studying ways to capture radioactive chemicals swimming in a sea of hazardous waste have created a new class of molecular cages that, like lobster traps, let certain species in while keeping others out. The new microporous materials, named Sandia Octahedral Molecular Sieves (SOMS) by their Sandia discoverers, could help purify industrial process or waste streams or filter out valuable chemicals for reuse. They also show particular promise for helping clean up the nation’s most pressing environmental problem, 53 million gallons of warm, radioactive muck inside 177 underground storage tanks at DOE’s Hanford, Wash., site — the byproduct of 50years of nuclear weapons production. (See “DOE’s underground storage tanks” on page 5.)The new SOMS are extremely selective forstrontium-90, one of the two most prevalent radioisotopes in the Hanford tanks.
In lab tests the SOMS trapped 99.8 percent of strontium-90 ions in parts-per-million concentrations from solutions containing chemically similar and highly abundant sodium ions, says Sandia principal investigator Tina Nenoff of Environmental Monitoring &Characterization Dept. 6233.The Sandia team is collaborating with researchers from the University of California –Davis (UC Davis), Pacific Northwest National Laboratory (PNNL), the University of Michigan, the State University of New York – Stony Brook (SUNY), and Lawrence Livermore National Laboratory(LLNL). The work is sponsored by DOE’s Environmental Management Science Program, which funds projects that provide options for reducing long-term DOE cleanup costs.
Picky ion catchers
Chemically, a SOMS is a tiny sponge that sucks up divalent cations (atom groups with a +2charge) into its microscopic pores and snares them at negatively charged bonding sites that have been vacated by ions with weaker charges — a process called ion exchange. (Home water softeners use ion exchange to remove iron from tap water.)
The SOMS are good ion exchangers because the sizes of openings on their crystalline surfaces can be adjusted precisely by altering the recipes followed to make them. By varying these openings from between 4 and 15angstroms (an angstrom is one ten-millionth of a millimeter), the researchers are able to select the sizes of ions or molecules that can get into the tunnels, and which can’t.When bound to water molecules in Hanford waste, for instance, strontium-90 is a fraction of an angstrom smaller than the million-times-more-abundant sodium-plus-water molecules. The result: Strontium is let in to the SOMS while sodium is kept out. “We can tune the pore size and the chemistry of the framework on the nano scale so the SOMS materials capture strontium on the bulk scale very selectively and efficiently, and in all types of environments,” says Tina.Because the SOMS are crystalline and inorganic, they also will stand up to the highly caustic environments found in the tanks.
Furthermore, when heated to about 500 ̊C, the strontium-saturated SOMS collapse into a dense glass-like material called a perovskite, its shrunken pores locking the strontium tightly into its crystalline structure.Bricks made from densified SOMS are impervious to leaching and stable against high pH, radiation, and heat, which might make them ideal for storage in high-level waste repositories such as the planned Yucca Mountain site, where the strontium could be immobilized until its radioactivity decays to safe levels — in perhaps 100 years.
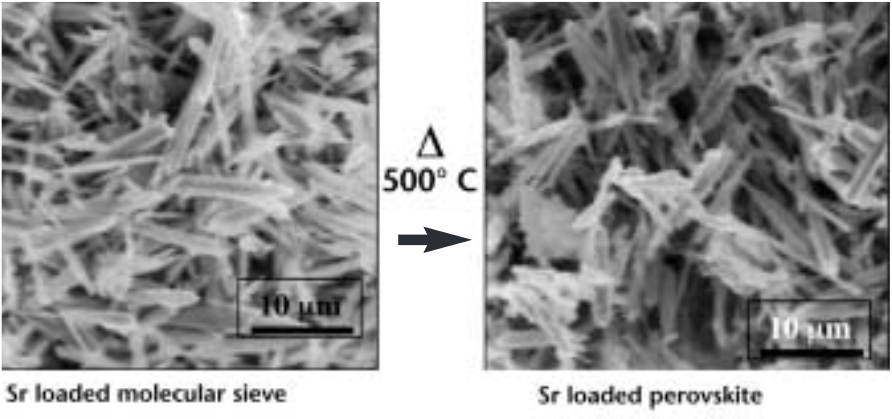
“This unique property gives SOMS the added bonus of being ready for the repository after only minimal processing,” she says.Liquefied tank waste could be pumped through columns containing the SOMS, she says. When the SOMS became fully loaded with strontium-90, they could be removed and densified for disposal.The Sandians discovered SOMS two years ago while characterizing the ability of another class of materials,crystalline sodium and cesium silicotitanates (CSTs), to selectively capture radioactive cesium-137 from waste streams at Hanford and then densify for long-term storage (Lab News, June10, 1994).(CSTs, developed in the early 1990s by a Sandia/Texas A&M/United Oil Products team, are now among DOE’s preferred cleanup technologies forcesium-137.)
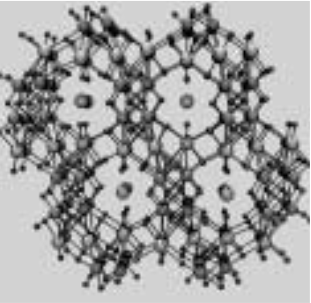
In synthesizing different phases of the CSTs, says Tina, “we came up with an entirely new class of crystalline materials that have no analogs in laboratory production or in nature. Not only are SOMS fascinating as a new material, they possess many useful properties.”Together, cesium-137 and strontium-90 makeup about 98 percent of the radioactive constituents in the Hanford tanks. Hanford’s tank-waste treatment plans include separating these radionuclides from the waste and disposing of them in a high-level waste repository.
Tina cautions that although the SOMS have been tested on waste simulants, they haven’t yet been tested on real waste, and there are several other technologies competing for the strontium-extraction job. Technically SOMS are sodium niobium oxide with transition metals such as titanium or zirconium added to give the SOMS their microporosity and ion exchange properties.
An eye on industrial uses
Although the SOMS team so far has focused on tank waste, the new microporous materials also could trap a variety of materials (such as chromium,cobalt, and nickel) in industrial waste streams.Once densified to a ceramic they could be useful in microelectronics fabrication and other industries where purification of, or extraction from, liquid process or waste streams is a significant or costly problem.
Sandia already is working with two mining-industry companies on using SOMS to extract and reuse valuable cobalt from copper-mine electrorefinement waste streams. The SOMS team includes Tina, May Nyman (both 6233), co-principal investigator Alexandra Navrotsky, Hongwu Xu (both UC Davis), co-principal investigator Mari Lou Balmer, Yali Su (both PNNL), Rodney Ewing (U. of Mich.), John Parise (SUNY), and Robert Maxwell (LLNL).
DOE’s underground storage tanks
Some 280 underground storage tanks at four DOE cleanup sites — Hanford, the Savannah River Site, Idaho National Engineering and Environmental Laboratory, and West Valley Demonstration Project in New York — contain a total of about 90 million gallons of mixed waste.Estimates put the cleanup tab for the Hanford tanks alone at more than $35 billion. And the clock is ticking. One-third of Hanford’s 177 tanks are leaking.The most critical wastes present in the Hanford tanks include radioactive isotopes of cesium, strontium, uranium, plutonium,technetium, and their decay products. The tank waste exists primarily in three forms — sludge, salt cake, and liquid.