First-of-their-kind snapshots reveal byproduct crippling powerful, experimental cells
For decades, scientists have tried to make reliable lithium-metal batteries. These high-performance storage cells hold 50% more energy than their prolific, lithium-ion cousins, but higher failure rates and safety problems like fires and explosions have crippled commercialization efforts. Researchers have hypothesized why the devices fail, but direct evidence has been sparse.

Now, the first nanoscale images ever taken inside intact, lithium-metal coin batteries (also called button cells or watch batteries) challenge prevailing theories and could help make future high-performance batteries, such as for electric vehicles, safer, more powerful and longer lasting.
“We’re learning that we should be using separator materials tuned for lithium metal,” said battery scientist Katie Harrison, who leads Sandia National Laboratories’ team for improving the performance of lithium-metal batteries.
Sandia scientists, in collaboration with Thermo Fisher Scientific Inc., the University of Oregon and Lawrence Berkeley National Laboratory, published the images recently in ACS Energy Letters. The research was funded by Sandia’s Laboratory Directed Research and Development program and the Department of Energy.
Internal byproduct builds up, kills batteries
The team repeatedly charged and discharged lithium coin cells with the same high-intensity electric current that electric vehicles need to charge. Some cells went through a few cycles, while others went through more than a hundred cycles. Then, the cells were shipped to Thermo Fisher Scientific in Hillsboro, Oregon, for analysis.
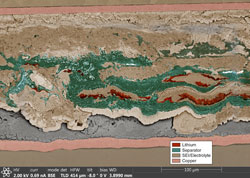
When the team reviewed images of the batteries’ insides, they expected to find needle-shaped deposits of lithium spanning the battery. Most battery researchers think that a lithium spike forms after repetitive cycling and that it punches through a plastic separator between the anode and the cathode, forming a bridge that causes a short. But lithium is a soft metal, so scientists have not understood how it could get through the separator.
Harrison’s team found a surprising second culprit: a hard buildup formed as a byproduct of the battery’s internal chemical reactions. Every time the battery recharged, the byproduct, called solid electrolyte interphase, grew. Capping the lithium, it tore holes in the separator, creating openings for metal deposits to spread and form a short. Together, the lithium deposits and the byproduct were much more destructive than previously believed, acting less like a needle and more like a snowplow.
“The separator is completely shredded,” Harrison said, adding that this mechanism has only been observed under fast charging rates needed for electric vehicle technologies, but not slower charging rates.
To learn more, read the complete news release.